Rigidifying cancer cells for better immunotherapy
Immunotherapy is a promising form of cancer treatment that boosts patients’ own T cells so that they can proliferate and destroy cancer cells. However, only about 20% of cancer patients are responsive to immunotherapy. Researchers have been working hard to increase this percentage by developing methods that can be combined with current immunotherapy to make the treatment effective in more cancer patients. “Our goal is always the same – to increase the percentage of patients who respond to immunotherapy,” says Prof. Li Tang, head of the Laboratory of Biomaterials for Immunoengineering, within EPFL’s School of Engineering. “One reason why the response rate is so low is that cancer is a very complex disease. We need to explore new approaches that, we hope, can target different aspects of the disease.”

Li Tang, Kewen Lei, and Armand Kurum © Alain Herzog / EPFL
Scientists at Tang’s laboratory have just developed one such approach that leverages biomechanics to fight against cancer. Their method increased the efficacy of immunotherapy considerably, opening up promising avenues for future research and translational applications. The study was a collaboration among EPFL scientists from Profs. Li Tang, Selman Sakar, and Georg Fantner groups, and a MIT research group headed by Prof. Ming Guo, and has just been published in Nature Biomedical Engineering.
Cancer cell camouflage
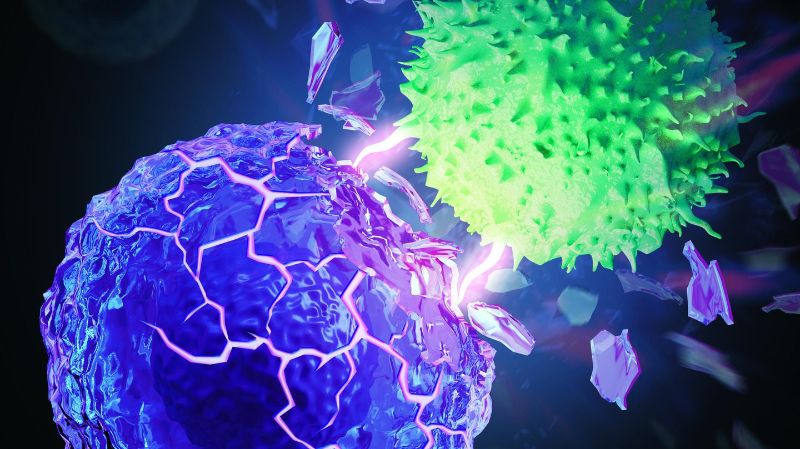
Kewen Lei and Armand Kurum, two PhD students in Tang’s laboratory, came up with the idea after observing that cancer cells are softer than healthy cells. The measurement of cancer cell mechanics is a challenging task, and the collaborations with Sakar’s, Fantner’s, and Guo’s laboratories contributed to solve this challenge. “As of today, researchers do not fully understand these changes,” says Lei. “While some scientists think that the softness of cancer cells make metastasis easier, that hasn’t yet been comprehensively demonstrated.” But now this new study shows clear evidence that the softness helps cancer cells evade T cell-mediated killing. T cells are fooled by the softness of cancer cells and pass by without attacking. “It’s a really tricky form of camouflage,” says Kurum. “When T cells attack a cancer cell, they push and pull on the cancer cell membrane. If the membrane is malleable, it provides less resistance and is therefore more difficult for the T cell to break.”
Stiffening cancer cells with an anti-cholesterol drug
Tang’s research group pinpointed what makes the cancer cell membranes soft: cholesterol, a lipid naturally present in cell membranes. The scientists developed a way to reduce the amount of cholesterol in cancer cell membranes using a common anti-cholesterol drug. By making the cell membranes stiffer, the drug makes it easier for T cells to destroy cancer cells. The anti-cholesterol drug was then combined with immunotherapy.

Stiffening cancer cells for enhanced T cell-mediated killing. © Kewen Lei / 2021 EPFL
The next step was to test their new method in preclinical models. The scientists administered the combined treatment to mice with melanoma tumors and compared the results with control groups. “The groups that received the immunotherapy only or the anti-cholesterol drug only did not survive the cancer,” says Tang. “But half of the mice that received the combination therapy survived. It indicates that giving the two treatments in tandem can increase the survival rate from 0% to 50% – a really encouraging discovery for future research.” The scientists now plan to combine their approach with other methods that can boost the performance of T cells in an effort to increase the immunotherapy response rate among cancer patients.
This multidisciplinary project could not have seen light without fruitful collaborations with laboratories specialized in the characterization of cell mechanics at EPFL (Prof. Sakar and Prof. Fantner) and MIT (Prof. Guo) and support from EPFL core facilities, including the Bioimaging and Optics Platform (BIOP), Center of Phenogenomics (CPG), and Flow Cytometry Core Facility (FCCF).