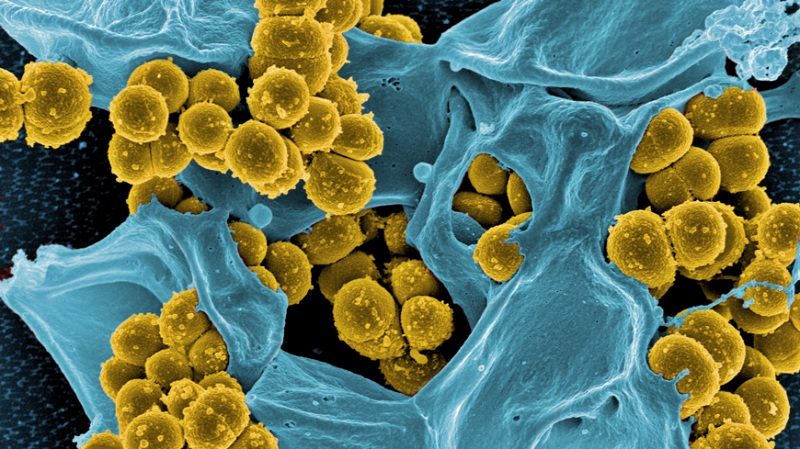
Rapid test for sepsis
For Qun Ren, every minute counts. The Empa researcher and her team are currently developing a diagnostic procedure that can detect life-threatening blood poisoning caused by staphylococcus bacteria rapidly. This is because staphylococcal sepsis is fatal in up to 40 percent of the cases. An infection with the spherical bacteria may have started as a local skin disease or pneumonia. Once the staphylococci have swarmed into the bloodstream in the course of sepsis, severe complications can arise. In such situations the pathogens must be identified as quickly as possible and appropriate antibiotics selected for treatment. This is particularly crucial for the survival chances of those affected, as Staphylococcus aureus strains can be insensitive to various antibiotics (see box). "If the bacteria in a blood sample first have to be cultivated for a diagnostic procedure, valuable time is lost," explains Qun Ren, the group leader from Empa's Biointerfaces lab in St. Gallen. Qun Ren and her team colleague Fei Pan therefore looked together with researchers from ETH Zurich for a way to bypass the lengthy intermediate step.
Fished out of the blood
The team has developed a method using magnetic nanoparticles that can bind to staphylococci. The bacteria can thus be specifically detected via a magnetic field. In a next step, the sensitivity to antibiotics is analyzed using a chemiluminescence method. If resistant bacteria are in the test tube, the sample emits light. If, on the other hand, the germs can be killed with antibiotics, the reaction vessel remains dark. "All in all, the sepsis test takes around three hours – compared to several days for a classic cultivation of bacterial cultures," says Fei Pan.
Dangerous glow
Another unpleasant representative from the bacterial kingdom is Pseudomonas aeruginosa. This rod-shaped bacterium can cause various diseases, including infections of the urinary tract, for example, via a urinary catheter during a hospital stay. Such infections can subsequently develop into sepsis. And these pathogens are also often resistant to a number of antibiotics.
This is where another advantage of the magnetic nanoparticles comes into play: The method can be tailored to many different types of bacteria, similar to a modular system. In this way, Empa researchers were able to develop a rapid "sepsis sensor" based on magnetic nanoparticles. In samples containing artificial urine, the method reliably identified the bacterial species and determined possible resistance to antibiotics via a chemiluminescence reaction.
So far, the researchers have evaluated their magnetic nanoparticle kit for sepsis and urinary tract infections using laboratory samples. "In a next step, we would like to validate the sepsis tests together with our clinical partners by evaluating patient samples," says Qun Ren.
Global antibiotic crisis
Worldwide, the declining effectiveness of antibiotics causes more than one million deaths each year. For example, some staphylococci can no longer be controlled with common antibiotics because they have developed resistance. The proportion of multi-resistant pathogens is particularly worrying. Already, the worldwide antibiotic resistance of pathogens is being described as a "silent pandemic." Depending on the country, for example, in Europe over 30% (Portugal, Italy) and around 1% (Scandinavia) of staphylococci are resistant to a wide range of antibiotics. In Switzerland, the number is currently 4.7%, according to 2021 statistics from the Federal Food Safety and Veterinary Office (FSVO). The bacterium Pseudomonas aeruginosa is also resistant to many antibiotics and can lead to severe pneumonia, urinary tract infections and sepsis. Therefore, when diagnosing an infection, the speed and precision, with which a germ is identified, can be critical for the survival of those infected.